Section 3 Water and Solutions Continued Answers
- Slides: 20
Download presentation

Solutions Section 3: Solubility and Concentration Preview • Key Ideas • Bellringer • Solubility in Water • Saturated Solutions • Concentration of Solutions • Math Skills

Solutions Section 3 Key Ideas 〉 What is solubility? 〉 What happens when you add more solute to a saturated solution? 〉 How do you describe how much of a solute is in a solution?
Solutions Section 3 Bellringer Many solutions can be found in your home. Most of these solutions contain several substances dissolved in a single solvent, such as water. The labels on products often indicate not only the ingredients that are in the solution, but also information about the concentration (the amount of solute in a volume of solution) of the main ingredients. Examine the labels on the next slide, and answer the items that follow.
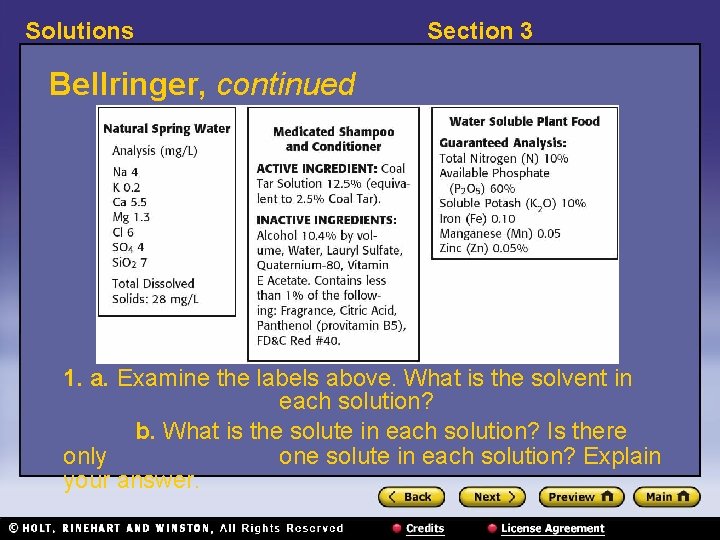
Solutions Section 3 Bellringer, continued 1. a. Examine the labels above. What is the solvent in each solution? b. What is the solute in each solution? Is there only one solute in each solution? Explain your answer.
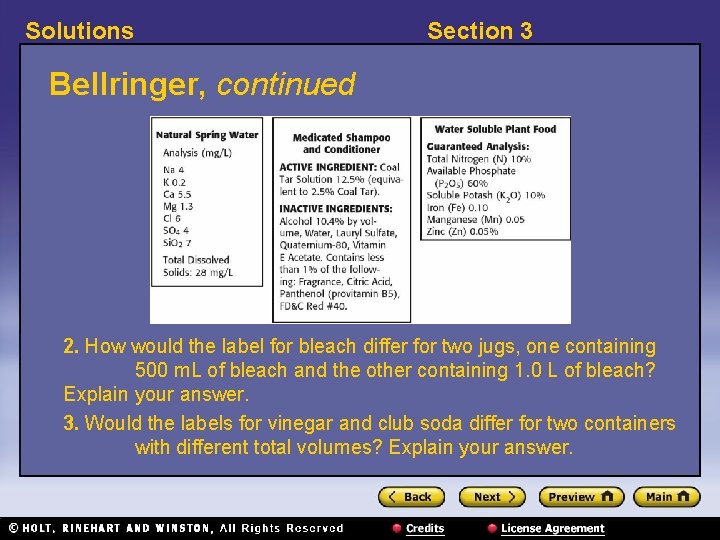
Solutions Section 3 Bellringer, continued 2. How would the label for bleach differ for two jugs, one containing 500 m. L of bleach and the other containing 1. 0 L of bleach? Explain your answer. 3. Would the labels for vinegar and club soda differ for two containers with different total volumes? Explain your answer.

Solutions Section 3 Solubility in Water 〉 What is solubility? 〉 The solubility of a substance is the maximum mass of a solute that can dissolve in 100 g of solvent at a certain temperature and standard atmospheric pressure. • solubility: the ability of one substance to dissolve in another at a given temperature and pressure
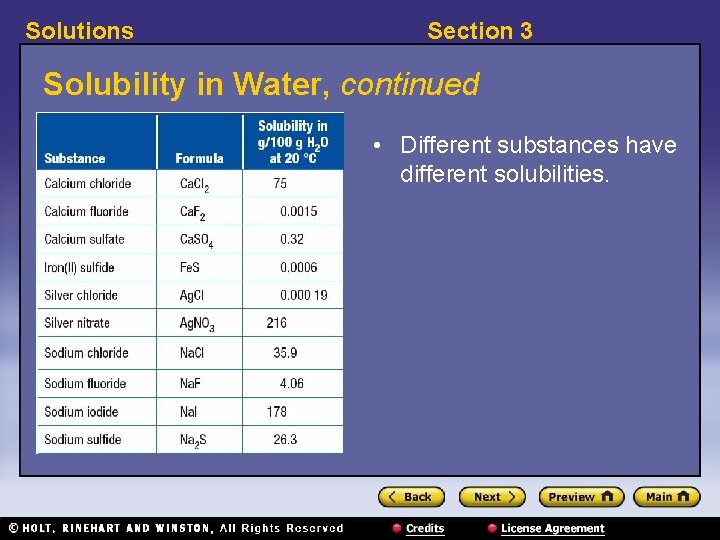
Solutions Section 3 Solubility in Water, continued • Different substances have different solubilities.
Solutions Section 3 Visual Concept: Solubility of a Solid in a Liquid Click the button below to watch the Visual Concept.

Solutions Section 3 Solubility in Water, continued • How much of substance is in a solution? • To express how much of a substance can dissolve in a solvent, you need to use the concentration. • Concentration: the amount of a particular substance in a given volume of a mixture, solution, or ore – A concentrated solution has a large amount of solute. – A dilute solution has only a small amount of solute.

Solutions Section 3 Saturated Solutions 〉 What happens when you add more solute to a saturated solution? 〉 In a saturated solution, the dissolved solute is in equilibrium with undissolved solute. So, if you add more solute, it just settles to the bottom of the container. • saturated solution: a solution that cannot dissolve any more solute under the given conditions

Solutions Section 3 Saturated Solutions, continued • Unsaturated solutions can become saturated. • unsaturated solution: a solution that contains less solute than a saturated solution does and that is able to dissolve additional solute • Heating a saturated solution can dissolve more solid. – The solubility of most solutes increases with temperature.

Solutions Section 3 Saturated Solutions, continued • supersaturated solution: a solution that holds more dissolved solute than is required to reach equilibrium at a given temperature. – To make a supersaturated solution, you raise the temperature of a solution, dissolve more solute, then let the solution cool again.

Solutions Section 3 Saturated Solutions, continued • Temperature and pressure affect the solubility of gases. • Gaseous solutes are less soluble in warmer water. • Example: Soda goes flat quickly at room temperature. • Gases are more soluble under higher pressure. • Example: When a can of soda is opened, carbon dioxide gas that had been dissolved in the soda bubbles out of solution.
Solutions Section 3 Visual Concept: Pressure, Temperature, and Solubility of Gases Click the button below to watch the Visual Concept.

Solutions Section 3 Concentration of Solutions ñ How do you describe how much of a solute is in a solution? ñ One of the most common ways of expressing the concentration of solution is molarity. • Molarity: a concentration unit of a solution expressed in moles of solute dissolved per liter of solution.

Solutions Section 3 Concentration of Solutions • Molarity is moles per liter of solution, not per liter of solvent. • A 1. 0 M solution of Na. Cl contains 1. 0 mol of dissolved Na. Cl in every 1. 0 L of solution.
Solutions Section 3 Visual Concept: Concentration Click the button below to watch the Visual Concept.

Solutions Section 3 Math Skills Molarity Calculate the molarity of sucrose, C 12 H 22 O 11, in a solution of 124 g of solute in 0. 500 L of solution. 1. List the given and unknown values. Given: mass of sucrose = 124 g volume of solution = 0. 500 L Unknown: molarity, amount of sucrose in 1 L of solution
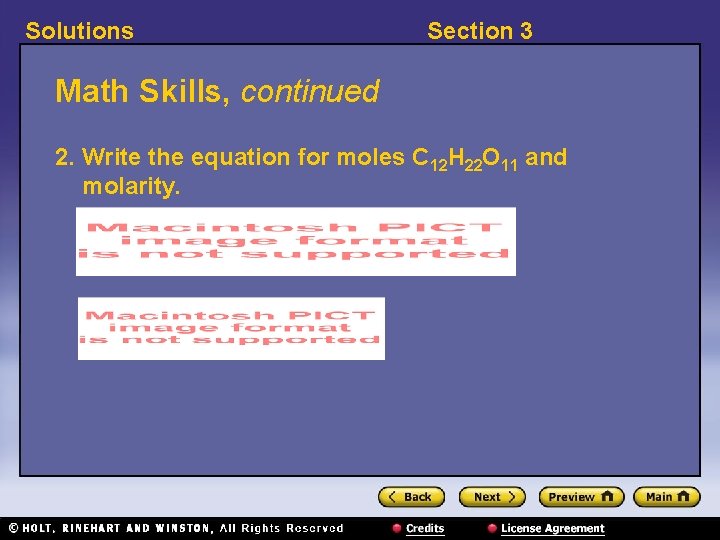
Solutions Section 3 Math Skills, continued 2. Write the equation for moles C 12 H 22 O 11 and molarity.
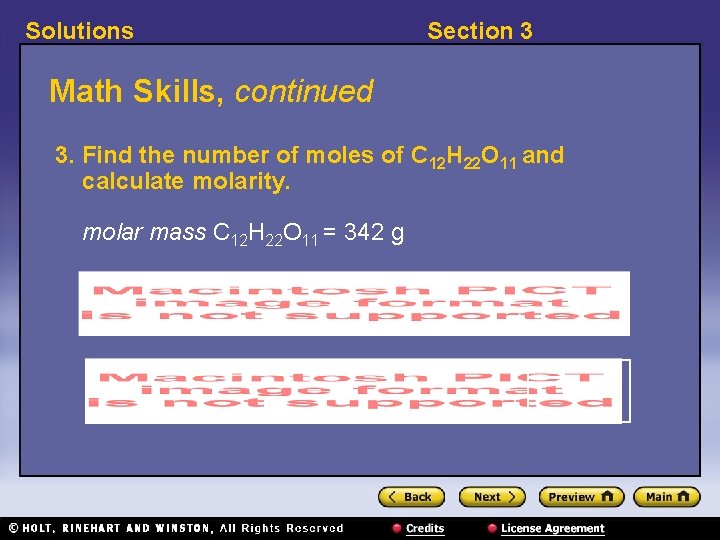
Solutions Section 3 Math Skills, continued 3. Find the number of moles of C 12 H 22 O 11 and calculate molarity. molar mass C 12 H 22 O 11 = 342 g
Source: https://slidetodoc.com/solutions-section-3-solubility-and-concentration-preview-key/
Post a Comment for "Section 3 Water and Solutions Continued Answers"